Author: Anna Aguilar
For the past seven years, the Spanish hospital organization where I am a Project Manager, Parc Taulí, has been involved in a relatively new way of promoting innovation in the public health care sector known as pre-commercial procurement (PCP) and public procurement of innovation (PPI). These complementary tools were set up by the European Commission to improve the efficiency of public services and stimulate competitiveness by means of innovative solutions acquisition.
Given our experience with PCP/PPI, it felt like a natural progression to get involved with the European Wide Innovative Procurement of Health Innovation (EURIPHI) project, whose key objectives were designed to build a foundation for future EU cross-border PCP/PPI applying a value-based approach. Our team views this EURIPHI partnership as an opportunity to learn more about these kinds of projects and to share the knowledge we have acquired since we started working on PCP/PPI projects in 2013.
Some highlights are described below. I explain PCP and PPI to provide context, followed by some of the core lessons we have learned over the years. These focus on the importance of clearly defining a procurer’s (hospital’s) unmet need in the preliminary tendering specifications prior to launching an open market consultation (OMC).
PCP and PPI explained
PCP is an EU-specific method for procuring research and development (R&D) services, using public needs as a driver for innovation. In the area of health, it provides a framework for consortia of health care procurers across Europe, which are all facing the same common challenge, to apply for a grant to jointly procure new R&D-based knowledge. This may later lead to innovative solutions addressing the shared unmet need.
In my opinion, this scheme can be advantageous to the European health care system, not least because public authorities and the private sector are both taking on the risks and benefits of the R&D.
In turn, PPI may be used by procurers when public health challenges can be addressed by innovative solutions that are nearly on the market. In this case there is no need for procurement of new R&D to bring solutions to the market; PPI is a tool to be utilized when there is a clear signal from a number of “early adopters” that they are willing to purchase and implement the innovative solutions under certain conditions.
In my opinion, procurer hospitals have the characteristics to facilitate these types of projects because, for example, they carry out clinical research that closely reflects the day-to-day clinical problems of health care professionals, they have a clear orientation towards innovation with involvement in collaborative work with companies in the sector, and they have capacity to mobilize professionals to achieve project success.
The European Commission sets out guidance for consortia on the whole PCP and PPI process, which includes, among other activities, drafting a prior information notice (PIN), OMC, preparing a request for tender document, and launching a call for tender. These activities take place during the various phases, which are described in the flow chart.
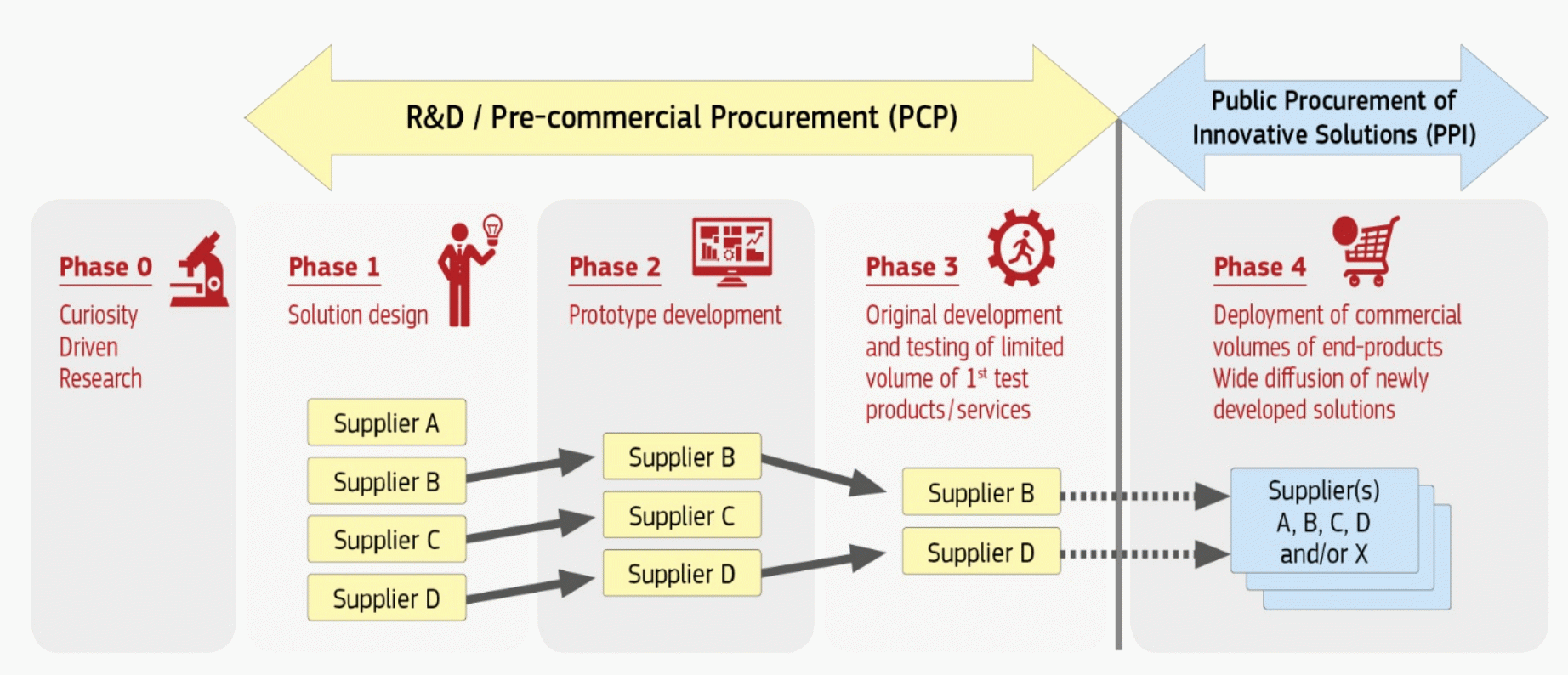
Source: European Commission (H2020 Programme: Guidance – PCP procurement documents)
Key considerations and challenges of PCP/PPI
As one of the first health care providers in Spain to become involved in PCP and PPI projects, Parc Taulí has faced a steep learning curve. Even though we have now participated in three PCP projects and two PPI projects (some completed and some ongoing), we still find that these types of projects are difficult to manage due to their complexity.
Despite the Commission’s clear mapping and organization of the PCP and PPI processes, it is up to the consortium partners to agree on how they will present the project specifications for which they are seeking an innovative solution, and of course to achieve a successful outcome. To this end, there are some key considerations and challenges.
Define the unmet need, not the solution
Early in the process, before the OMC, the consortium must prepare an internal preliminary request for tender document describing its objectives and clearly defining the unmet need – not a product or service – for which it is seeking an innovative solution. After the OMC, the request for tender document is finalized, and the call for tender is published.
Therefore, it is essential for the consortium to be very specific about what it wants to buy, so that companies that want to bid on the tender will have a strong understanding of the need and challenges. We have often found, however, that this is the most difficult task because health care professionals, who know what the clinical problem is and see it daily, tend to focus on the solution rather than on the unmet need.
I cannot emphasize enough that the procurers must identify the need, while the companies must find a potential solution.
For example, Parc Taulí is one of five leading hospitals that is currently working on a PCP to address patient stress management before, during, and after surgery (Patient Empowerment by Stress Avoidance and Recovery Services, or STARS). The consortium agreed that the main need is “reducing stress and improving health condition of patient ongoing surgery during complete care path”. We then had to outline the R&D services required, such as “improvements in the quality of life of patients and improvement of working conditions of caregivers and clinicians”.
To define the unmet needs and functional requirements of the solution, we had weekly meetings with the buyers’ group of the consortium. In these meetings we discussed these functional requirements to prioritize which requirements the solution should have, and which requirements would be nice to have. This is a long process, so consortium partners must be sure to allocate enough time for it.
Take a multidisciplinary approach
Because we need to think about the problem that we want to solve as an organization, it is critical to have a multidisciplinary team (project managers, IT department, innovation technicians, clinicians, etc.) participating in the project from the outset. This is key to having a transversal vision of the project and to specify this view in the document.
We have certainly learned from our mistakes: In our first PCP project, we did not have a good grasp of the process, so we started working with only the clinicians. We later encountered problems when the IT department told us that the solution could not be implemented due to a lack of resources. Had we sought input from all stakeholders at the beginning, we could have avoided this.
A multidisciplinary approach is also essential when defining the award criteria (e.g., the level of originality and innovativeness of the proposed solution and ability to go beyond the current state of the art), as well as the weights that will apply during assessment for all phases. Here it is crucial to think about value-based procurement and not only about the price of the solution.
Carefully plan your open market consultation
As part of the preparation for an innovation driven procurement action, a PIN will be published to pre-inform suppliers about the planned procurement and invite them to the OMC. The PIN – released at least 30 days before publication of the call for tender – describes how the market consultation sessions are conceived and organized. The OMC is critical because it is an opportunity to find out if companies understand the health care procurer’s needs and challenges.
From my perspective, it is very important to have a good OMC dissemination campaign to reach large number of companies. For the STARS project, we carried out several OMCs in five different locations.
Be aware of language & legislative barriers
The language and legislation of the different countries participating in the project can be an unexpected barrier to the project’s smooth running. When there are multiple countries participating in the project, procurers should be aware that the legislation followed is that of the country coordinating the project. At every step of the project, it is therefore necessary to be in touch with the coordinator to ascertain whether an activity is permitted within their legislative framework.
Benefits of PCP/PPI
Apart from the difficulties, I still believe that the advantages of utilizing PCP/PPI outweigh the disadvantages. One positive aspect is the mobilization of professionals at hospitals during the project, from the Managing Director, to the clinicians, the IT department, the procurement department, the innovation department, and the financial department.
This creates a real innovation culture in the hospital where people share information between departments and learn new concepts and ways to buy solutions. Everybody is aligned on the project, which encourages great synergies that result in better outcomes for patients.
From a broader perspective, these types of projects allow hospitals to open opportunities for collaboration with companies to enhance innovation in the health sector, provide opportunities to small- and medium-sized enterprises to scale-up for foreign markets, and ultimately benefit European citizens.

Anna Aguilar has a degree in Statistics from the Autonomous University of Barcelona, Marketing Management Postgraduate and Project Management training by La Salle Barcelona. She has more than 8 years of experience in project management and she is currently working as a Project Unit Coordinator at Fundació Parc Taulí in the health sector. She has expertise at National and International level and she has been involved in several European projects and in three of FP7 – H2020 Pre-Commercial Procurement (PCP) projects: NYMPHA-MD, THALEA and STARS. She is currently involved in two H2020 Public Procurement of Innovation Solutions (PPI) projects: THALEA II and EcoQUIP+. Her expertise in international project management includes the financial management and auditing of the projects.
EURIPHI has been an opportunity for Parc Taulí to take part of an European network of Innovative Procurement of Health, to learn more about value based procurement and the mode of cross-border cooperation and also to share our experience and knowledge in PCP and PPI.




